Good Manufacturing Practices or GMP is a system that consists of processes, procedures and documentation that ensures manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards. Implementing GMP can help cut down on losses and waste, avoid recall, seizure, fines and jail time. Overall, it protects both company and consumer from negative food safety events.
GMPs examine and cover every aspect of the manufacturing process to guard against any risks that can be catastrophic for products, such as cross-contamination, adulteration, and mislabeling. Some areas that can influence the safety and quality of products that GMP guideline and regulation address are the following:
·Quality management
·Sanitation and hygiene
·Building and facilities
·Equipment
·Raw materials
·Personnel
·Validation and qualification
·Complaints
·Documentation and record keeping
·Inspections & quality audits
What is the difference between GMP and cGMP?
Good Manufacturing Practices (GMP) and current Good Manufacturing Practices (cGMP) are, in most cases, interchangeable. GMP is the basic regulation promulgated by the US Food and Drug Administration (FDA) under the authority of the Federal Food, Drug, and Cosmetic Act to ensure that manufacturers are taking proactive steps to guarantee their products are safe and effective. cGMP, on the other hand, was implemented by the FDA to ensure continuous improvement in the approach of manufacturers to product quality. It implies a constant commitment to the highest available quality standards through the use of up-to-date systems and technologies.
What are the 5 Main Components of Good Manufacturing Practice?
It is paramount to the manufacturing industry to regulate GMP in the workplace to ensure consistent quality and safety of products. Focusing on the following 5 P’s of GMP helps comply with strict standards throughout the entire production process.
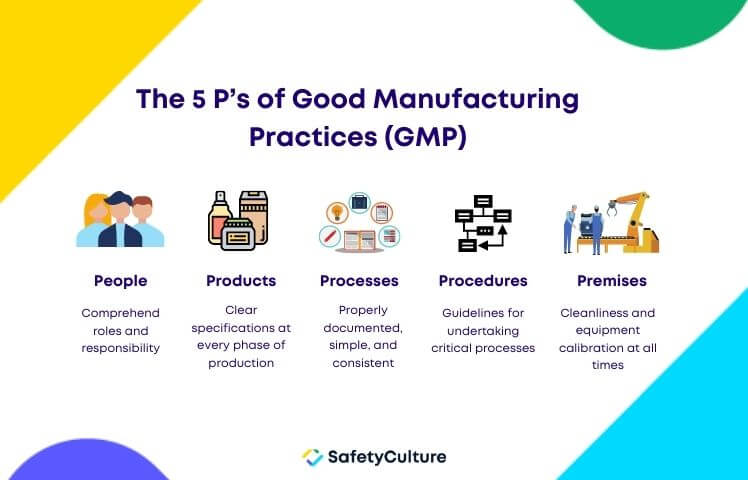
The 5 P’s of GMP
1. People
All employees are expected to strictly adhere to manufacturing processes and regulations. A current GMP training must be undertaken by all employees to fully understand their roles and responsibilities. Assessing their performance helps boost their productivity, efficiency, and competency.
2. Products
All products must undergo constant testing, comparison, and quality assurance before distributing to consumers. Manufacturers should ensure that primary materials including raw products and other components have clear specifications at every phase of production. The standard method must be observed for packing, testing, and allocating sample products.
3. Processes
Processes should be properly documented, clear, consistent, and distributed to all employees. Regular evaluation should be conducted to ensure all employees are complying with the current processes and are meeting the required standards of the organization.
4. Procedures
A procedure is a set of guidelines for undertaking a critical process or part of a process to achieve a consistent result. It must be laid out to all employees and followed consistently. Any deviation from the standard procedure should be reported immediately and investigated.
5. Premises
Premises should promote cleanliness at all times to avoid cross-contamination, accidents, or even fatalities. All equipment should be placed or stored properly and calibrated regularly to ensure they are fit for the purpose of producing consistent results to prevent the risk of equipment failure.
What are the 10 Principles of GMP?
1. Create Standard Operating Procedures (SOPs)
2. Enforce / Implement SOPs and work instructions
3. Document procedures and processes
4. Validate the effectiveness of SOPs
5. Design and use working systems
6. Maintain systems, facilities, and equipment
7. Develop job competence of workers
8. Prevent contamination through cleanliness
9. Prioritize quality and integrate into workflow
10.Conduct GMP audits regularly
How to Comply with GMP standard
GMP guidelines and regulations address different issues that can influence the safety and quality of a product. Meeting GMP or cGMP standards helps the organization comply with legislative orders, increase the quality of their products, improve customer satisfaction, increase sales, and earn a profitable return of investment.
Conducting GMP audits play a big part in assessing the compliance of the organization to manufacturing protocols and guidelines. Performing regular checks can minimize the risk of adulteration and misbrand. A GMP audit helps improve the overall performance of different systems including the following:
·Building and facilities
·Materials management
·Quality control systems
·Manufacturing
·Packaging and identification labeling
·Quality management systems
·Personnel and GMP training
·Purchasing
·Customer service
Post time: Mar-29-2023